
Introduction: A Tiny Patient, A Giant Leap for Medical Science
In a landmark achievement that resonates with hope and heralds a new era in medical science, an infant born with a life-threatening genetic disorder has become the first patient in the world to receive a personalized gene-editing treatment using CRISPR technology. This groundbreaking intervention, carried out by a dedicated team at the Children’s Hospital of Philadelphia (CHOP) and Penn Medicine, offers a profound glimpse into the future of personalized medicine and the potential to conquer devastating rare diseases.
The case of KJ Muldoon, a baby boy who faced a grim prognosis, now stands as a testament to the power of scientific innovation and collaborative research, offering a beacon of hope to countless families worldwide grappling with similar challenges.
This article delves into the details of this extraordinary medical first, exploring the nature of the genetic disorder, the revolutionary CRISPR treatment administered, the journey of young KJ, and the broader ethical and future implications of this pioneering approach. The successful treatment, documented in The New England Journal of Medicine, not only signifies a personal victory for KJ and his family but also marks a pivotal moment in the ongoing quest to harness the power of our own genetic code to heal and transform lives (CBS News, 2025; Children’s Hospital of Philadelphia, 2025).

Understanding CPS1 Deficiency: A Rare and Devastating Genetic Disorder
Carbamoyl Phosphate Synthetase 1 (CPS1) deficiency is a rare, autosomal recessive genetic disorder that falls under the umbrella of urea cycle disorders. The urea cycle is a critical metabolic pathway primarily occurring in the liver, responsible for converting toxic ammonia – a byproduct of protein metabolism – into urea, which is then safely excreted from the body in urine. In individuals with CPS1 deficiency, the gene responsible for producing the CPS1 enzyme is mutated. This enzyme plays a crucial first step in the urea cycle. Without a functioning CPS1 enzyme, ammonia accumulates rapidly in the bloodstream, leading to a condition known as hyperammonemia.

The CRISPR Revolution: Tailoring Gene Editing for a Single Patient
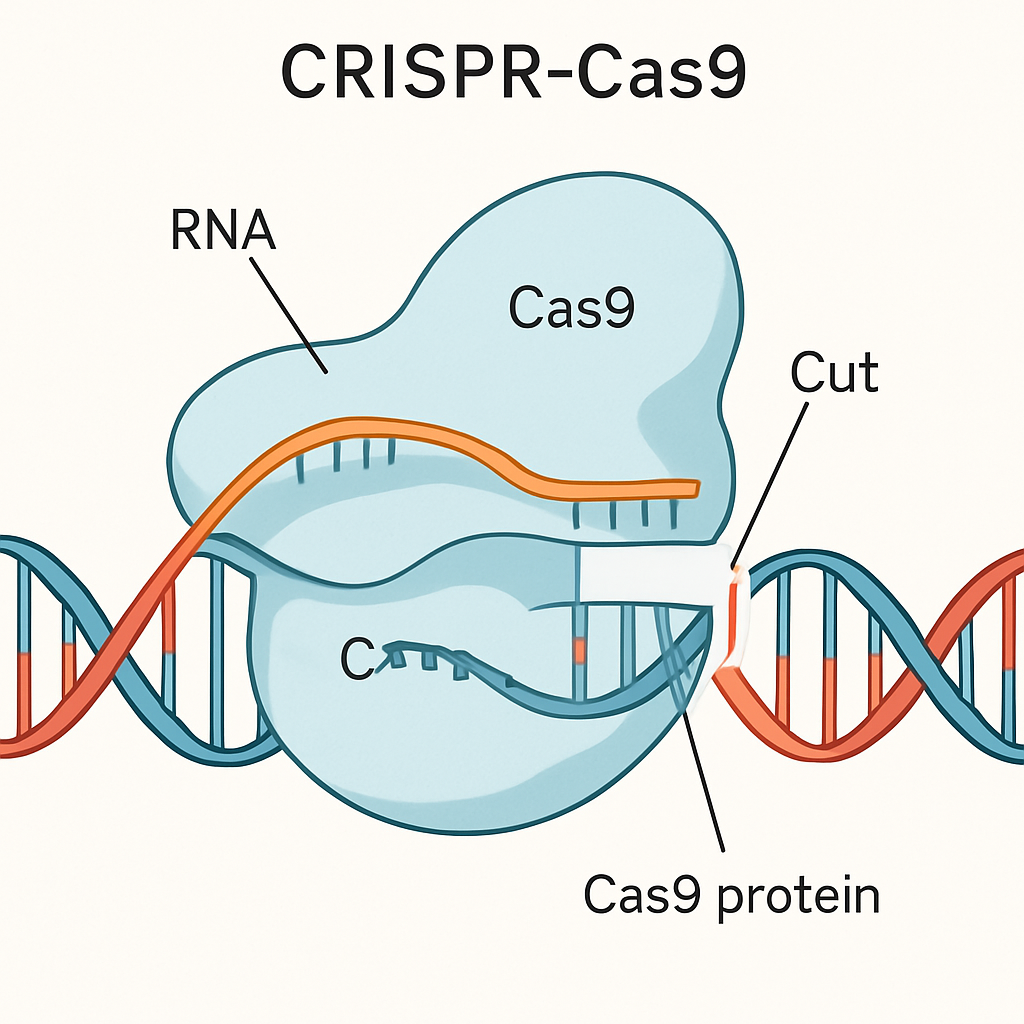 Conceptual illustration of the CRISPR-Cas9 system editing a DNA strand. This technology allows for precise modifications to the genetic code.
Conceptual illustration of the CRISPR-Cas9 system editing a DNA strand. This technology allows for precise modifications to the genetic code.A Glimmer of Hope: Early Results and KJ's Progress

Ethical Considerations and Future Horizons: Navigating a New Medical Frontier
Conclusion: A New Chapter in Genetic Medicine, Written with Courage and Innovation

References
- CBS News. (2025, May 15). Infant becomes world’s first patient to undergo personalized gene-editing treatment. Retrieved from https://www.cbsnews.com/news/infant-worlds-first-patient-personalized-gene-editing-treatment-crispr-liver-disorder/
- Children’s Hospital of Philadelphia. (2025, May 15). World’s First Patient Treated with Personalized CRISPR Gene Editing Therapy at Children’s Hospital of Philadelphia. Retrieved from https://www.chop.edu/news/worlds-first-patient-treated-personalized-crispr-gene-editing-therapy-childrens-hospital
- Innovative Genomics Institute. (n.d.). CRISPRpedia: What is CRISPR? Retrieved from https://innovativegenomics.org/crisprpedia/ (Note: This is a general reference for CRISPR explanation, specific IGI involvement in KJ’s case is through the Somatic Cell Genome Editing Consortium mentioned in CHOP press release).
- Musunuru, K., Ahrens-Nicklas, R., et al. (2025). Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease. The New England Journal of Medicine. Published online May 15, 2025. DOI: 10.1056/NEJMoa2504747 (as cited by Children’s Hospital of Philadelphia).
- The New York Times. (2025, May 15). Baby Is Healed With World’s First Personalized Gene-Editing Treatment. (Snippet reference for CPS1 incidence rate, full access was limited). Retrieved from https://www.nytimes.com/2025/05/15/health/gene-editing-personalized-rare-disorders.html

